STEM stand for Science, Technology, Engineer and Math. For me Science and Technology is the hardest subjects in my life. My teacher always have something know for us to learn and it really interesting.In first round week five, I learn about Physical Science. In our class our teacher divided into four teams to present and teach each other about our topic. There are four topics, which are Proton, Neutron and Electron / Atomic, Force and Quark / Ion and Isotope / Atomic Mass and Atomic Number. I am in Ion and Isotope team. In my team, I have four members. I am research about Ion and my other member research about Isotope. I have been doing the research of Ion for one day and a half. Now let me explain Ion and Isotope that I get and learn from my research. Ion is an atom with a net charge that loss or gain of one or more electrons. It can create charge because the Proton and Electron are not balancing. Ion form when atom gain or lose electrons. It form is also related to Ionization energy and the position on the periodic table. Ionization energy is the amount of the energy that remove the electrons from atom. There are two types of Ionization energy, low and high. Low Ionization energy means that it easy to loss the electrons. High Ionization energy means that it easy to gain the electrons. There are two types of Ion. Positive Ion we call Cation. It happened when atom lose the electrons.Negative Ion we call Onion. It happened when atom gain the electron. We also can find Ion in Humans Body. It’s play the important role in Humans Body. There are some Ion keys that participate in Humans Body. There are Calcium, Potassium, Sodium, Chloride and Copper. For Sodium is the positive Ion that found in the fluid outside the cell. Potassium is the positive Ion inside the cell. Isotopes are atoms that have the same number of protons and electrons but different number of neutrons. The different number of Isotope have different mass number. If an atom were to gain or lose Neutrons it becomes an Isotope. The atom that gain Neutrons call Deuterium.

Let’s move on to Proton, Neutron and Electron. From I understand from my teammates Neutron have zero charge, Electron have negative charge and Proton have plus charge. The positive charge stay in Nucleus. Neutrons help combine the atom in oxygen. Proton and Neutron almost have the same size. Proton and Electron always have the same amount. Neuton helps repelling Proton apart. Proton and Neutron always stick with each other. Now I will explain about Quark, Atomic and Force. There are four types of Atomic force Strong Force, Electromagnetic Force, Weak Force and Gravitational Force. I only know what Electromagnetic Force mean. The other three I’m not sure. So Electromagnetic Force mean it’s how it repel when negative plus positive. For Example : When you have two magnet and you put positive and positive or negative and negative together it will stick with each other, but if you put positive and negative or negative and positive together it will strongly push each other out and it can’t stick with each other. There are six types of Quarks, Up quarks, Down quarks, Strange quarks, Charm quarks, Top quarks and Bottom quarks. When You have Down quarks, Up quark and Down quark you will get Neutron. Scientist keep Quark as the smallest thing in universe. Mostly the color of Quark is light color like Green, Blue, Red and etc. It’s uncountable.
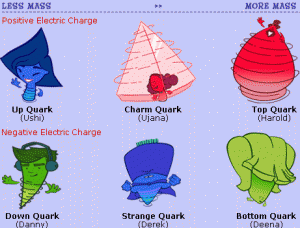
The last one is Atomic Number and Atomic Mass. Atomic Number is the number in the corner of the formula. If you want to see the neutrons of Atomic you just need to round atomic mass and subtract with atomic number and you will see. That’s all I get to know from my friend and from what I research.


